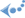Novel Drug Form of Chlorin E6
1 institute of Biomedical Chemistry of Russian Academy of Medical Sciences, 19832, Pogodinskaya str., 10, Moscow, Russia,
2 N.N. Blokhin All-Russian Cancer Research Center of Russian Academy of Medical Sciences, 115478, Kashirskoye shosse, 24, Moscow, Russia,
3 ChemBridge Corporation, Moscow Division, 119435, Malaya Pirogovskaya str., 1, Moscow, Russia,
4 JSC "Veta", 111395, Alleya Pervoi Mayovki, 15, Moscow, Russia.
ABSTRACT
Keywords: photodynamic therapy, photosensitizer, chlorin х6, chlorins, drug form, drug substance, photocytotoxiciry, cytotoxicity.
INTRODUCTION
The most well-known chlorin photosensitizers (PSs) are derivatives of chlorin e6. It has already been shown l, 2, 3 that some of them favour the tumor-to-muscle ratios of c.a. 10 in experiments with tumor-bearing animals after i.v. injections of 5-200 mg kg-1 doses.
Easily obtained from pheophorbide-a in alkaline conditions4, clorin e6, was by itself well biologically studied 5, 6, 7. It has been known to be suitable for PDT for long. However, to our knowledge, there were no reports about clinical applications of chlorin e6, mainly because of difficulty of its high-scale production from biological substrates. General problems have been known to be short shell lives of the ready drug forms in solutions, poor solubility of the dry sodium salt forms and a bad antibacterial filtration ability of such solutions8. Nevertheless, its usefulness as a photosensitizing part of photoimmunotoxines for targeted PDT9-13 as a synton for further chemical modification14-21 , and as a promising PS5-7, 21 has been widely recognized.
Chlorin х6 drug substance was produced by JSC "Veta" Ltd, Moscow, Russia. Its 1% sterile solution containing 0.5% of N-memyl-D-glucosamine as solubilizing and stabilizing agent (drug form "Photodithazine") was prepared at the Laboratory of drug form preparation of the Research institute of experimental tumor diagnostics and treatment of N.N.Blokhin All-Russian Cancer Research Center of Russian Academy of Medical Sciences.
As references here were used the only two drugs permitted for the clinical use in Russia - oligomerized hetnatoporphyrin-IX mixture "Photogem" (Russia) prepared at Moscow state academy of fine chemical technology at the Department of chemistry and technology of fine organic compounds headed by Prof. A.F.Mironov, and sulfonated aluminium phthalocyanine complex "Photosense" (Russia) prepared at the State research center of organic semiproducts and dyes, both of them being supplied from the clinics of N.N.Blokhin All-Russian Cancer Research Center of Russian Academy of Medical Sciences.
PREPARATION OF A STABLE, WELL SOLUBLE
AND FILTRATING FORM OF CHLORIN e6
 There is
a patented procedure at our disposal now allowing for preparation of a
stable, well water soluble and filtrating form of chlorin e6
- "Photodithazine"23
(Fig. 1). The main feature of the molecule is the type of its
counter-ion - N-methyl-D-glucosamine - in accordance with our statement
that to stabilize big ions of PSs in aqueous media as well big
counter-ion(s) are needed.
There is
a patented procedure at our disposal now allowing for preparation of a
stable, well water soluble and filtrating form of chlorin e6
- "Photodithazine"23
(Fig. 1). The main feature of the molecule is the type of its
counter-ion - N-methyl-D-glucosamine - in accordance with our statement
that to stabilize big ions of PSs in aqueous media as well big
counter-ion(s) are needed.The elaborated production technology allows for "Photodithazine" drug substance standard series derived from pure pheophorbide-a, where contents of the active component exceed 95%. The technology includes 4 steps: (1) acetone extraction of chlorophyll a from dry biomass of cyanobacteria Spirulina Platensis, its demetallation with diluted hydrochloric acid and purification of the resulting pheophytin a; (2) acid hydrolysis of the latter in organo-aqueous medium to pheophorbide-a and its purification; (3) saponification of pheophorbide-a to chlorin e6, its conversion to its N-methyl-D-glucosamine complex, purification by gel-chromatography and (4) antibacterial Millipore filtration and preparation of drug form "Photodithazine".
The most characteristic features of this technology are centrifugal purification in each step and a careful approach to step 3, when pheophorbide-a is treated with alkali. It is crucial to provide such conditions for the reaction to avoid the oxidation (allomerization) reactions at carbon C-132 of the exocyclic ring of pheophorbide-a. This last requirement must be met during the previous steps as well. Otherwise, another derivative of 17,18-dihydroporphyrin series is predominantely obtained, namely purpurin 18, transforming in alkaline conditions to only poorly soluble in water chlorin p6
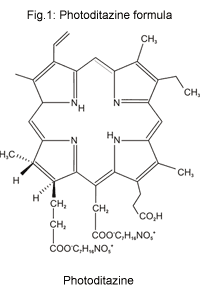
The long-wave part of "Photodithazine's" electronic absorption spectrum is given in Fig.2.
Fig.2: A part of "Photodithazine's" absorption spectrum for concentration 26 mM/ml in 0.01 M borate buffer with human serum albumine addition (1%).
2-OCTANOL/PHOSPHATE BUFFER PARTITION
COEFFICIENT DETERMINATION
Table 1: 1-Octanol/phosphate buffer, pH 7.4 partition coefficients.
|
Photosensitizer |
Kp |
|
Photoditazine |
1.4 |
|
Photogem |
1.5 |
|
Photosense |
0.05 |
CYTOTOXICITY AND PHOTOCYTOTOXICITY
METHODS
Cytotoxic and photocytotoxic studies of "Photodithazine" have been performed in vitro using PC 12 cells (rat pheochromocytoma).
In PC12 test cells were seeded on 48 well dishes and maintained in 0.3 ml RPMI 1640 medium (Flow, UK) without phenol red, supplemented with 10% fetal calf cerum, and gentamycin. All the dishes were kept in the dark at 37░C in a humidified and containing 5% carbon dioxide atmosphere and in black paper. MTT, 3-[(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide] (Sigma), was dissolved in PBS, filter sterilized and stored at 4░C. The PSs were added to the wells to the final concentrations of 0.5, 1, 2.5, 5, 10, 50 and 100 uM and incubated with the cells for 3h for spontaneous redistribution throughout them. There were three special controls in both light and dark experiments: (1) without MTT (with crystal violet dye), (2) with MTT, but without PS, and (3) with PS, but without MTT.
Dishes for light experiments were then irradiated with laser light: lex 633 nm (helium-neon laser) for "Photogem", and lex 670 nm (SHG YAP:2 laser) for the others at the doses of 20 J/cm2.
After the photoirradiation, the dishes were incubated for 39 h. MTT solution (150 ul, 0.5%) was added to each well and incubated for 4 h at 37░C to allow for MTT metabolization. The crystalls formed were dissolved within one night by addition of 300 ml per well 25% SDS solution and vigorously pipetted to ensure homogenicity of solution prior to scanning or using a shaker overnight. Then PBS solution was added to each well (50 ml, pH 7.4). The absorbances at 530 nm were measured using a Bio-Tek Instruments Multiscan plate reader. The results were compared with the control wells and expressed with respect to the control values". The high PS concentrations of 50 and 100 mM were found to carry in a big error in the subsequent formazane absorbance measurements due to their own absorption at 530 nm, and this is why the optical density of the wells was corrected by distraction the value of optical density of control wells (with PS, but without MTT).
For every PS two experiments were done: with photoirradiation (лlight╗) and without photoirradiation (лdark╗), and in each pair of the dishes there was found to be an equal amount of cells. There were 118,400 cells in each well for "Photodithazine", 107,500 ones for "Photosense", and 137,600 ones - for "Photogem" as determined by an assay with dye crystal violet. Using crystal violet the number of cells was determined the following way27. The cell monolayer was stained with 0.2% crystal violet in 2% ethanol. After the staining cells were washed with water and the dye was eluted with 10% acetic acid. Number of the cells was calculated from the optical density of the eluate at 550 nm, the optical density (in 1 cm cuvettes) of 0.1 corresponding to c.a. 32,500 cells.
The amount of cells was determined to introduce a correction coefficient between experiments with different PSs (different dishes, seedings, etc.). Moreover, through crystal violet it became possible to find a-cpefficient to determine the amount of living cells by indirect way using MTT test and to follow the quantity of living cells at MTT test, because it was shown that both in light and in dark experiments the two methods gave similar results.
The results of the photocytotoxicity assay are shown in Table 2 and Fig. 3.
Table 2: Photocytotoxicity assay results in terms of EC50 and EC65.
|
PS efficacy/ cell culture
|
EC50, UM |
||
| Photoditazine | Photogem | Photosense | |
|
pheochromocytoma |
- | 3 | 5 |
|
PC12 |
5 (EC65) | 45 (EC65) | 25 (EC65) |
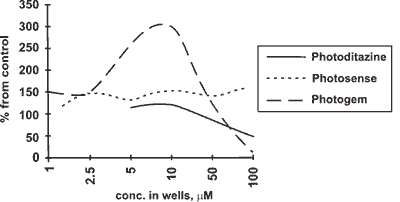
Fig.3: Photocytotoxicity curves for PC12 cells
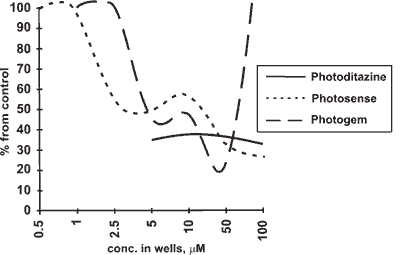
The results of the assay in respect of PC 12 test are shown in Fig 4. Cytotoxicity in this case was found only at the concentrations over 50 (UM, and it appeared to be that "Photogem" was even more cytotoxic than "Photodithazine" a i\ there was no cytotoxicity of "Photosense".
Fig.4: Cytotoxicity curves for PC 12 cells
DISCUSSION
As regards the photocytotoxicity assay, if we exclude extremely low and high concentrations in PC 12 test, and see the range of 10-50 mM, then the PSs will make up the following sequence: "Photogem" > "Photodithazine" > "Photosense" (Fig.3). It is, however, worth mentioning that "Photosense" was most photocytotoxic at the low and very high concentrations. Generally speaking, PC 12 test seemed to us quite reliable, because cell deth occuring after 1.5-3 h incubation prior to photoirradiation is known to come mainly from plasmatic membrane damage, and the more lypophylic PS is, the more likely plasmatic membrane will be its primary target. So, "Photogem" and "Photodithazine" are close in their photocytotoxicity, with "Photosense" being less photocytotoxic.
At the low and very high concentrations tetrapyrrol PSs are observed to possess somewhat unexpected behavior, acting as either cell proliferation promoters or specific activators of some enzimes. This observation (done by other groups, too, e.g. J.-L.Merlin et. al. showed that an increase in both light fluence and HPD extracellular concentration resulted in a decrease in cytotoxicity [28]) may partly be proved by "Photogem" dark curve (Fig.4), supposing that with photoirradiation the zone of лpromoter proliferations or лenzime activation╗) concentrations moves to the periphery of the scale. We observed this effect with many other porphyrins, and this is, probably, due to formation of some activated species after or during the photoirradiation, that this moving occurs. This effect seems to overlap with photocytotoxicity effects, making them Jook so poor in the periphery of the light curves. We would rather suppose that this was for dehydrogenase enzimes activity increase, because we could see in fact the amount offonnazane crystalls go up inctead of increasing the amount of cells. Besides, there was no such лproliferation╗ effect observed in the middle of the scale (10 mM) with CaOv cells, to which DNA synthesis inhibition method was applied.
From this point of view, in PC 12 test "Photosense" was лactivating╗ through the whole scale, "Photogem" -extremely лactivating╗ below 50 mM, "Photodithazine" was practically characterized by none of the effects below 50 mM, with both "Photodithazine" and "Photogem" being cytotoxic in the dark at 50-100 mM concentrations.
Thus, PC 12 cell test support the prognosis of Kp determination in terms of amphiphilicity, and there is a good correlation between Kp values and photocytotoxicity of PSs in the concentration range of 5-50 mM with PC 12 cells.
CONCLUSIONS
Photocytotoxic properties of "Photodithazine" have been evaluated, and it was supposed that its high photodynamic efficacy was connected also with its ability to penetrate into biological membranes. It did not show any toxicity on PC 12 cell culture without irradiation except the range of very high concentrations.
ACKNOLEDGEMENTS
REFERENCES
2. K.Aizawa, T.Okunaka, H.Kawabe, Y.Yasunaka, S.O'Hata and T.Saito, ((Localization ofmono-L-aspartyl chlorin хс (NPe6) in mouse tissues╗, Photochem. Photobiol. 46, 789-793 (1987).
3. C.Gomer, A.Ferrario, лTissue distribution and photosensitizing properties ofmono-L-aspartyl chlorin хс; in a mouse tumor model╗. Cancer Res. 50, 3985-3990 (1990)
4. S.Lotjonen, P.H.Hynninen, лA convenient method for the preparation of chlorin es and rhodin g7 trimethyl esters╗, Synthesis, 541- 543(1980).
5. K.Iwai, S.Kimura, T.Ido and R.Iwata, лTumor uptake of [48V] vanadyl-chlorine хс Na as a tumor-imaging agent in tumor-bearing mice╗. Nuclear Med. Biol. 17, 775-780 (1990).
6. G.P.Gurinovich, T.E.Zorina, S.V.Melnov, N.I.Melnova, I.F.Gurinovich, L.A.Grubina, M.V.Sarzhevskaya and S.N.Cherenkevich. лPhotodynamic activity of chlorin хс and chlorin хс ethylenediamine in vitro and in vivo╗, J. Pholochem. Photobiol. B. Biol. 13, 51- 57(1992).
7. G.A. Kostenich, I.N. Zhuravkin, E.A. Zhavrid, лExperimental Grounds for Using Chlorin E(6) in the Photodynamic Therapy of Malignant Tumors╗, J. Photochem.Photobiol.B-Biol.ll, No. 3, 211-217(1994).
8. K.M. Smith, S.-J. Lee, лLong-wavelength Water Soluble Chlorin Photosensitizers Useful for Photodynamic Therapy and Diagnosis of Tumors╗, Pat. 5,330.741 (07/1994) USA/ C.A., 119, 244560z (1994).
9. A.R.Oseroff, D.Ohuoha, T.Hasan, J.C.Bommer and M.L.Yarmush, ((Antibody-targeted photolysis: selective photodestruction of human T-cell leukemia cells using monoclonal antibody-chlorin хс conjugates)), Proc. Nail. Acad. Sci. USA. 83, 8744-8748 (1986)
10. B.A. GofF, M. Bamberg and T. Hasan, лPhotoimmunotherapy of human ovarian carcinoma cells ex vivo╗. Cancer Res. 51,4762-4767(1991),
11. ┬ A Goff, U.Hermanto, J. Rumbaugh, J. Blake, M Bamberg, T.Hasan, лPhotoimmunotherapy and biodistribution with an ╬╤ 125-chlorin immunoconjugate in an in vivo murine ovarian cancer model╗, Br. J. Cancer., 70, No.3, 474-480 (1994).